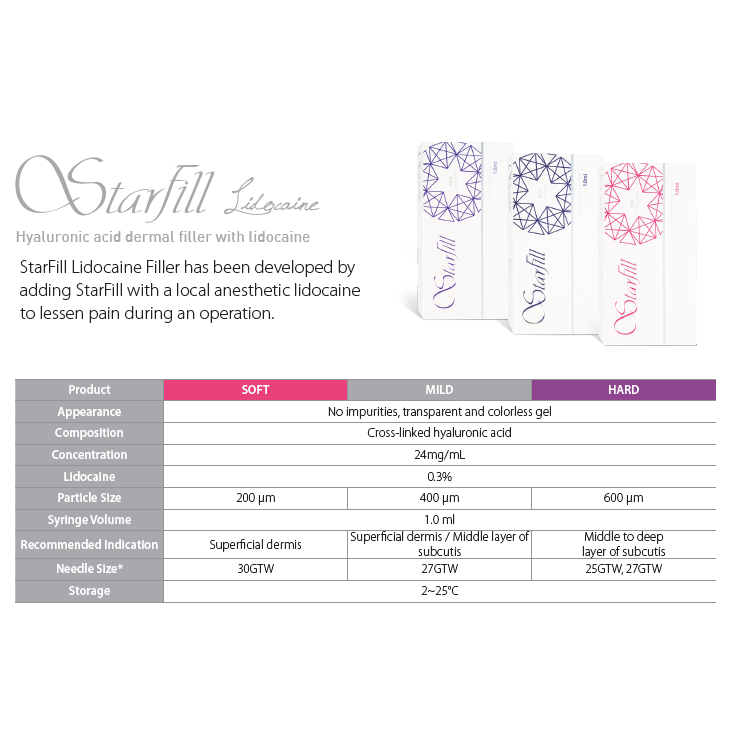
Description:
Starfill fillers are obtained using the “Zero Endotoxin and BDDE Entire Process” (ZEEP) technology, which guarantees minimized endotoxin and residual BDDE levels. The product has high HA cross-linking degree, outstanding viscoelasticity and cohesiveness parameter values. Low toxin and cross-linking agent levels ensure high safety of the product while minimizing the risk of post-injection edema, allergic reactions, and other side effects. Uniform gel particle size contributes to even distribution of the filler during injection and better aesthetic effect. The presence of lidocaine in the composition makes the procedure painless.
Strengths of StarFill:
the series contains products varying in the gel particle size and HA cross-linking rate depending on the scope of use endotoxin level is below 0.1 EU/ml
no residual BDDE even distribution of the gel during injection due to uniform particle size high viscoelasticity and cohesiveness result in longer aesthetic effect duration
proven effectiveness as demonstrated by the Wrinkle Severity Rating Scale (WSRS) results of the assessment of wrinkle improvement on week 8, 16, and 24 after the injection procedure
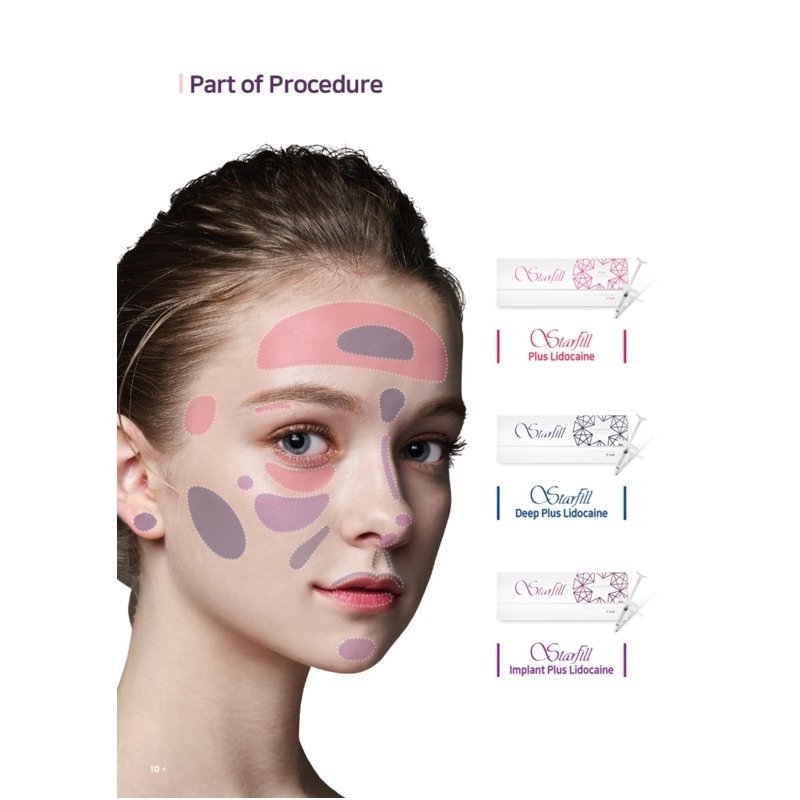
StarFill Deep Plus Lidocaine is used for correction of moderately pronounced and deep facial lines and wrinkles. The filler is injected into the mid and deep dermis.
Scope of StarFill Deep Plus Lidocaine:
-nasolabial folds
-forehead “worry” and glabellar “frown” lines
-chin crease
-perioral wrinkles (marionette lines, smoker’s lines, mouth frown)
-lip contouring and augmentation
The aesthetic effect lasts from 9 to 15 months.
Product composition: Cross-linked sodium hyaluronate 24 mg/ml, Lidocaine hydrochloride 3 mg/ml, PBS
StarFill Deep Plus Lidocaine
1 syringe × 1.1 ml, 2 needles per pack
Needle size: 27G



.png)
.png)
.png)


.png)
.png)

.png)
.png)
.png)
.png)